
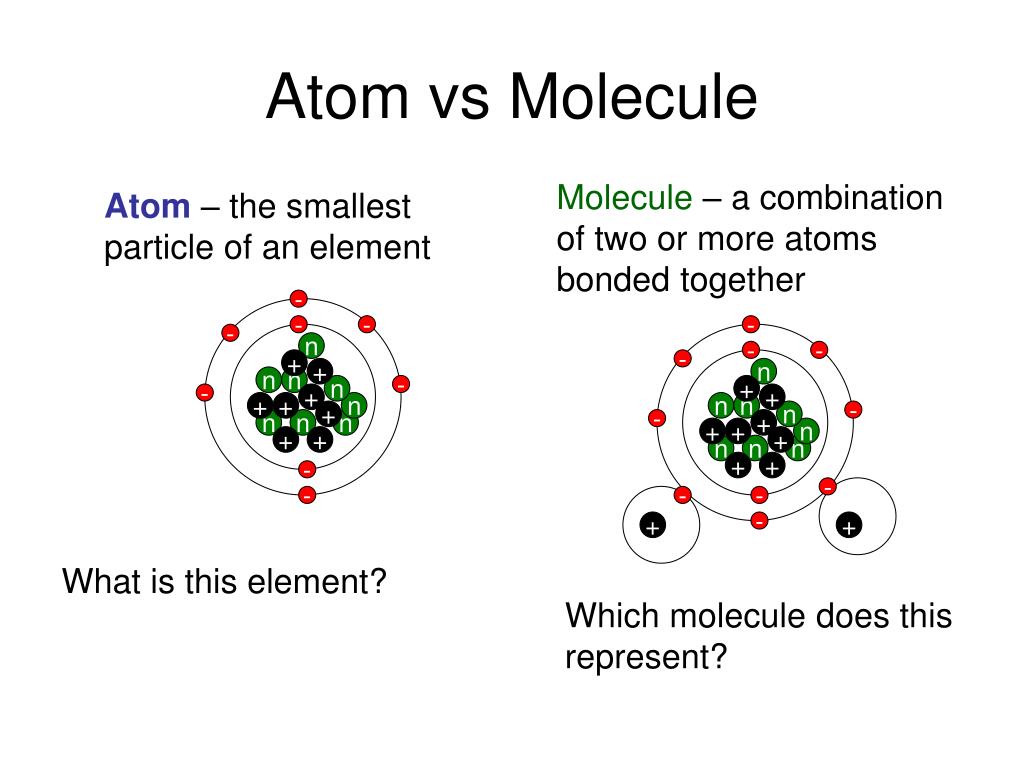
C is the atomic symbol for carbon and can represent not only the element, (but one or more) atoms of the element, as well as the element as it exists in the understood (implied) conditions. Oxygen isn't always O 2 but can be formed (in the upper atmosphere or in some reactions) as O 3.Ĭhemists mix and match their terminology somewhat freely when it doesn't matter much, but try to be as specific as possible when it does.Ĭontext is important. But, if you are talking about reactions, it is usually worth describing the molecular form of the element you are talking about. It matters little which version you use to describe the element. We might talk about sulfur as S or, if we care about the allotrope we might specify S 8, though there are others common in the lab. Carbon, however, is usually found as a solid and never as a simple molecule (diamond and graphite are both covalently bonded solids) sort is rarely useful to describe its normal molecular form as there isn't one. Nitrogen is also mostly found in pure form as a diatomic gas (N 2).
#Atom vs molecule free
Since this is by far the commonest way we find free oxygen in nature we often describe it this way anyway unless there is a reason not to. Oxygen is usually found as a diatomic gas (which is why we write O 2). Then it isn't enough to describe just the element, we need to know something about how it is found under normal conditions. But sometimes we need to describe how the element appears in the world or in chemical reactions. When we are just talking about the element, then just using the symbol by itself is clear.


Sometimes we use terminology a little loosely.


 0 kommentar(er)
0 kommentar(er)
